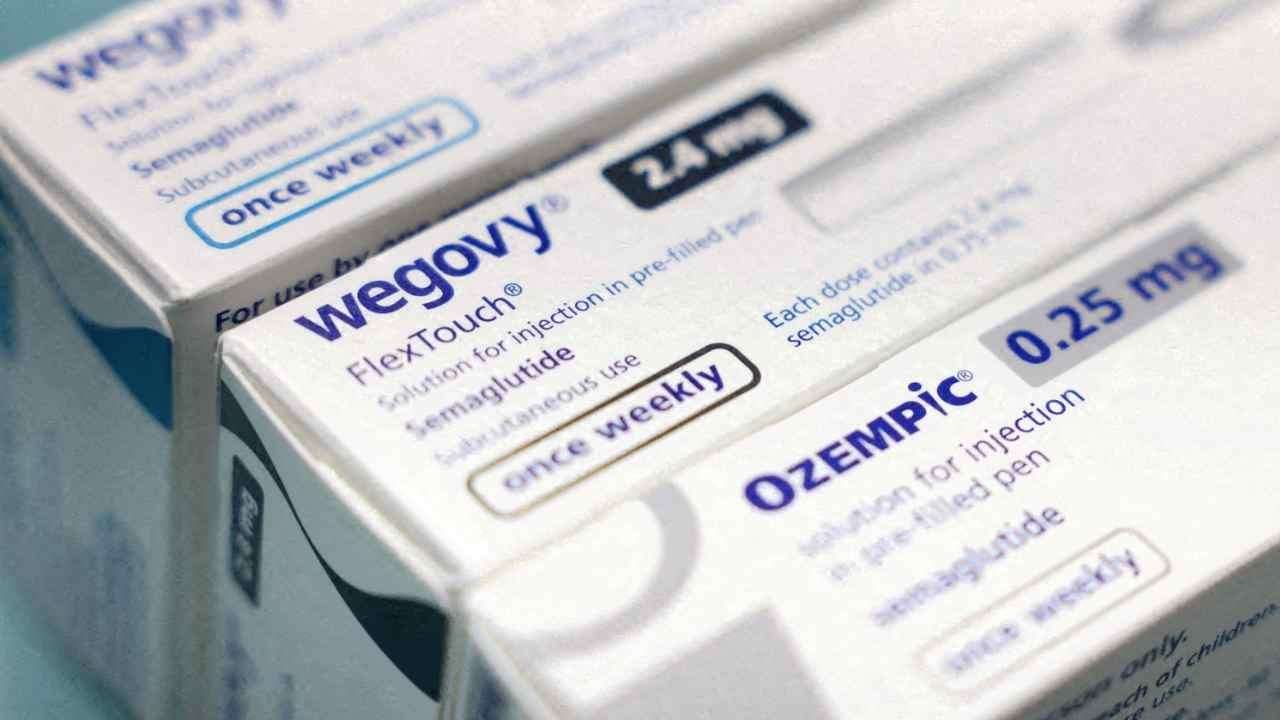
US health regulators have approved the first pill version of the blockbuster weight-loss drug Wegovy, marking a major milestone in obesity treatment. The decision by the US Food and Drug Administration gives Novo Nordisk a competitive advantage over rival Eli Lilly, whose oral obesity drug, orforglipron, is still awaiting approval.
Both medications belong to the GLP-1 class of drugs, which work by mimicking a natural hormone that suppresses appetite and increases feelings of fullness—similar to widely used injectable treatments.
Novo Nordisk’s injectable Wegovy and Eli Lilly’s Zepbound have already transformed obesity care in the US and globally, where obesity affects roughly 100 million Americans. Company officials said the Wegovy pill is expected to reach the market within weeks, potentially expanding access by offering a more convenient and possibly lower-cost alternative to injections.
Although about one in eight Americans have used injectable GLP-1 drugs, many struggle with the high cost. Experts say oral options could help close that gap. “There’s a large group of patients who could benefit from pills,” said Dr Fatima Cody Stanford, an obesity specialist at Massachusetts General Hospital.
The new Wegovy pill contains 25mg of semaglutide—the same active ingredient used in Wegovy and Ozempic injections, as well as the diabetes pill Rybelsus. In clinical trials, participants taking the oral version lost an average of 13.6% of their body weight over roughly 15 months, compared with a 2.2% loss among those given a placebo. Injectable Wegovy has shown slightly higher average weight loss of around 15%.
One trial participant, Chris Mertens, a pediatric lung doctor from Wisconsin, said he lost about 40 pounds while taking the pill. He reported reduced appetite and fewer intrusive thoughts about food.
Eli Lilly’s oral drug orforglipron showed an average weight loss of 11.2% over nearly 17 months at its highest dose. However, both pills were less effective than Lilly’s injectable Zepbound, which targets two gut hormones and has produced average weight loss of about 21%.
Side effects across GLP-1 drugs—whether oral or injectable—are similar and include nausea and diarrhea. While both pills offer daily convenience, Novo Nordisk’s version must be taken on an empty stomach with a small amount of water, followed by a 30-minute wait before eating or drinking. This requirement is due to special formulation measures that protect the drug during digestion.
Lilly’s pill, by contrast, has no dosing restrictions and is under review through the FDA’s priority voucher program, with a decision expected by spring.
Manufacturing pills is typically cheaper than producing injectable drugs, raising hopes for lower prices. The Trump administration previously said it had negotiated reduced pricing for GLP-1 medications, which can cost more than $1,000 per month. Novo Nordisk said its starting dose pill will be available for $149 per month through select providers, with more pricing details to be released in January.







.svg)
